
GUIDOR® easy-graft:
The Handling Advantage
Simplifying bone regeneration techniques In their forward thinking 2003 article (1), Hammerle & Jung stated:
“Developments in bone augmentation procedures can be related either to simplification of the clinical handling or to influencing of biological processes. To simplify clinical handling, new materials should comprise a matrix with optimal cell ingrowth capacities and good mechanical properties, providing space for tissue regeneration. No membrane and no specific procedures for mechanical fixation should be necessary. This would reduce the technique sensitivity and increase the predictability of bone augmentation. The use of synthetic (alloplastic) materials would result in lower surgical risks and lower morbidity in augmentation procedures and
would represent an important step forward in simplifying bone regeneration techniques.”…
Minimally invasive surgery and site-specific access flaps
New tools to split the crest are now available, including bone expanders and the piezoelectric scalpel. With such tools, the idea of a reduced or flapless approach to implant dentistry, alleviating post treatment side effects, acce-l erating healing and avoiding bone resorption caused by periosteal elevation becomes feasible…
…with GUIDOR easy-graft ® and its stunningly easy handling such considerations become reality.
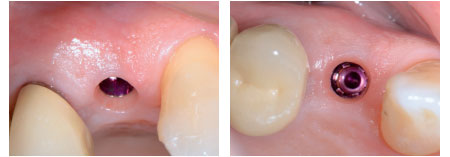
“Minimally invasive” and “Immediate-delayed”
implant protocols are ideal cases for mouldable bone graft materials with in-situ hardening.
GUIDOR easy-graft®:
Trend-Setter
1/4 million applications since 2007
Launched in Switzerland following collaboration with Zurich University and then Internationally in 2007, GUIDOR easy-graft® has quickly established itself as a leading alloplastic material with over 250,000 applications. GUIDOR easy-graft® products are available in two resorption formats.
Both offer:
• 100% Alloplastic material(no substances of animal or human origin)
• High porosity
• Osteoconductivity
• Syringe application
• Mouldable, in situ hardening
The GUIDOR easy-graft® principle:

GUIDOR easy-graft®:
Mouldable from the Syringe, in-situ Hardening

GUIDOR easy-graft®:
Resorption Process for BioLinker® and Polymer Coating
The resorption processes for BioLinker® and PLGA polymer coating takes place in two stages:

Stage 1) BioLinker® is extracted within hours
BioLinker® makes easy-graft® CLASSIC and easy-graft®CRYSTAL mouldable. In the defect, BioLinker® is extracted by incoming blood, promoting rehardening of the material. More than 90% of BioLinker® is removed from the bone graft substitute within three hours (1) and excreted through the urine within 1–3 days (2). BioLinker® contains NMP which is widely used in pharmaceutical and medical devices such as dental membranes, subcutaneous drug-release systems etc.
Resorption process for PLGA polymer coating

Stage 2) The polylactide coating (PLGA) is resorbed over a few weeks.
In parallel to the healing and regeneration process the PLGA coating and adhesive connection between the granules gradually weakens (three to six weeks in vitro), exposing the osteoconductive scaffold. PLGA polymers are widely used in devices such as membranes, screws and plates for maxillofacial surgery, suture anchors, and cages for spinal surgery. Resorption of PLGA releases small amounts of lactic and glycolic acid. Lactic acid is found naturally in the body and is degraded by metabolic processes. Glycolic acid is a fruit acid and can be degraded in the body or excreted with the urine.
GUIDOR easy-graft®:
Tips and Tricks
Handling (fig 1)
Retracting the plunger makes wetting of the granules with BioLinker® easier.
Condensing in the defect (fig 2)
If possible, GUIDOR easy-graft® products should be condensed in the defect for an intimate contact between the material and bone. The granules are pressure-stable and abrasion-resistant, which prevents potentially inflammatory debris from forming. When applied to large areas the material can be moulded and then compressed (aseptic conditions) with the finger using moist gauze.
Dispensing the material (fig 3)
GUIDOR easy-graft® products can be dispensed on a sterile dry surface after mixing with a dry instrument (aseptic conditions) and placed in several small defects during a single surgical procedure. The material does not harden until in contact with body fluids, consequently, this method can be used for filling multiple small bone defects in one patient with one single 0.4ml application.
Flexible, porous shell (fig 4)
After mixing, GUIDOR easy-graft® products can be (aseptically) shaped into a thin shell between two sterile, smooth and dry surfaces after addition of a few drops of sterile water or sterile saline solution. The flexible, porous shell can, for example, be used for fixing and as resorption protection for autogenous bone chips or as a cover in an external sinus lift.
Direct bone contact
PLGA absorbs small amounts of water which creates a slight volume increase thus improving the graft to surrounding bone tissue contact. In some cases the patient may have a slight feeling of pressure in the first few days after application and should be advised accordingly. When filling the defect the material should be condensed and not overflow the margin of the bone defect.
Reentry (fig 5a, 5b)
Depending upon time scale, visualization of granules embedded on the surface of new bone will be observed. When undertaking flapless or minimally invasive (membrane-free) surgery excess granules can sometimes be seen in the soft tissue. Granules in the soft tissue can easily be removed at reentry if desired.

GUIDOR easy-graft®:
Quick Summary







